FDA Business Registration Services
- Consult
- National Service Hotline :
0755-27391220

0755-27391220
服务内容 / Service Content
According to the US FDA requirements, all manufacturers (including OEM processors) exporting finished and semi-finished medical devices to the US market should register their enterprises in the US FDA system.
After successful registration, the company will get Registration Number, FEI Number and Owner/Operator Number.
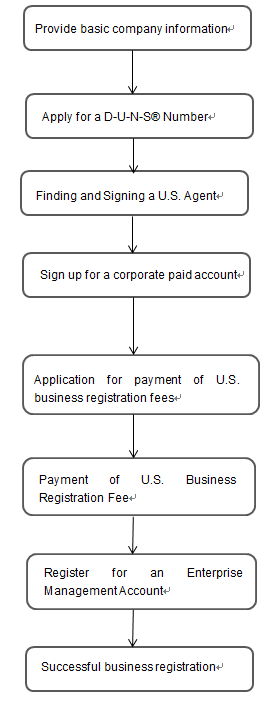
FDA medical device enterprise registration process is:
Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
xxxx@email.com