MDL/MDEL Registered Agent Service in Canada
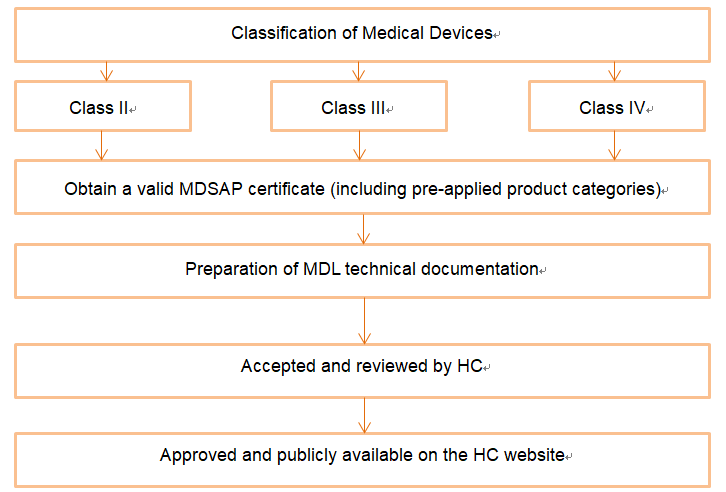
When a product is determined to be a medical device in Canada, it is necessary to submit information to Health Canada (Health Canada) for registration; it is worth noting that it is sufficient to apply for an MDEL for Class I medical devices in Canada, and an MDL for Class II, Class III, and Class IV (the applicant must have a certificate of MDSAP accreditation).
- Consult
- National Service Hotline :
0755-27391220