Australia TGA Registered Agent Service
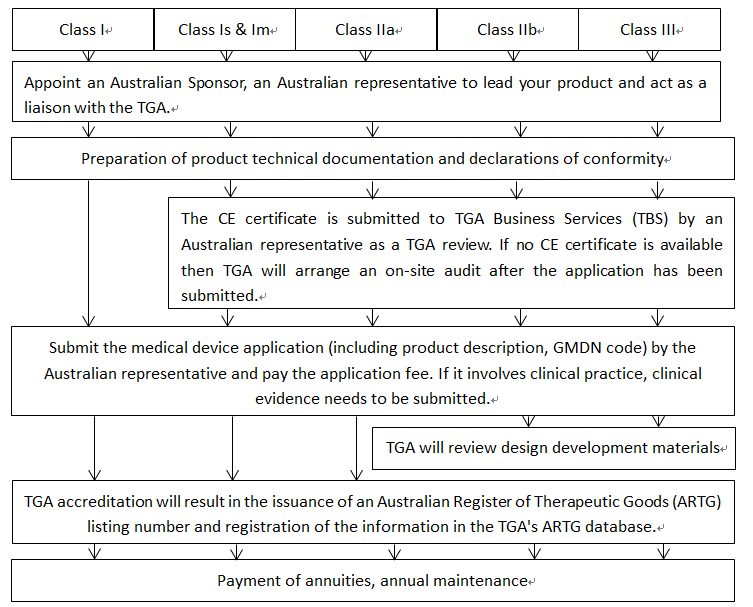
TGA registration in Australia, TGA is short for Therapeutic Goods Administration, the full name of the Therapeutic Goods Administration, which is the oversight body for therapeutic goods (including medicines, medical devices, genetic technologies and blood products) in Australia. the TGA carries out a range of accreditation and supervisory and regulatory work to ensure that therapeutic goods supplied in Australia meet applicable standards and to ensure that therapeutic levels of the Australian community The TGA conducts a range of review and regulatory activities to ensure that therapeutic commodities provided in Australia meet applicable standards and that the Australian community's level of therapeutic
The regulatory framework is designed to determine the management of public health and safety, while at the same time relieving businesses of any unnecessary regulatory burdens. In fact, the regulation requires that any product must be registered through the Australian Register of Therapeutic Goods (ARTG) before it can be manufactured or sold in Australia. ARTG is a computerised database of approved products that have been found to meet human safety requirements. ARTG is a computerised database of approved products that have met human safety requirements.
- Consult
- National Service Hotline :
0755-27391220