Japan PMDA Registered Agent Service
- 立即咨询
- 全国服务热线:
0755-27391220
服务内容 / Service Content
Medical device companies wishing to market their products in Japan must comply with Japan's Pharmaceutical and Medical Device Act (PMD Act).
Japan's Ministry of Health, Labour and Welfare (MHLW), the Pharmaceuticals and Medical Devices Agency (PMDA) is the regulatory body in Japan. The PMDA (Pharmaceuticals and Medical Devices Agency) is the regulatory body in Japan.
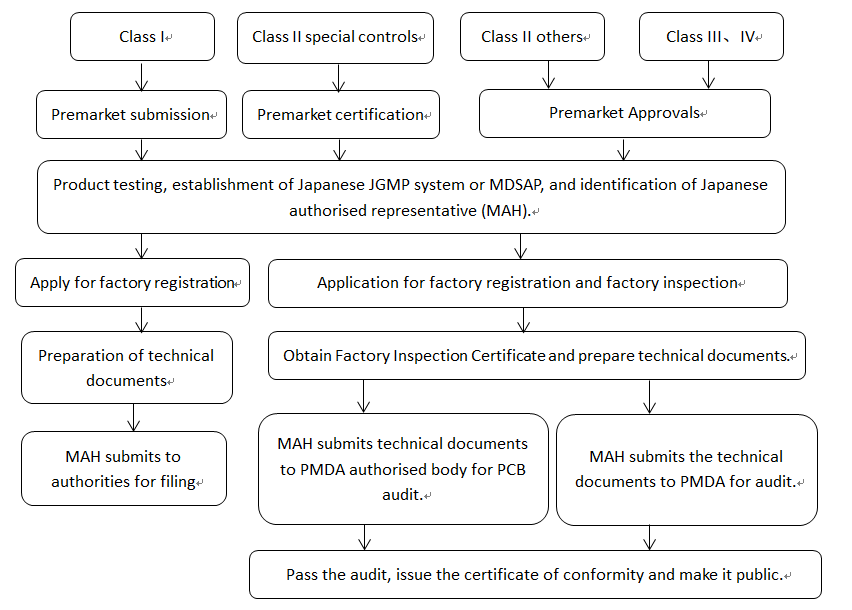
Medical devices must be registered by their Marketing Authorisation Holder (MAH) through the following procedures.
Class I Devices - Pre-market Todokede Submission
A Class I device must be submitted by the MAH or DMAH to the PMDA with a pre-market submission that is not subject to review and approval by the PMDA.
Class II Devices - Premarket Approval Ninsho
Pre-market certification is required before marketing a Class II device as a special control. A Certification Body (PCB) is an organisation authorised by PMDA to carry out PMDA certification.
Class II, III,IV Devices - Pre-market Approval Shonin
Except for specially controlled Class II devices, Class II devices and Class III,IV devices must have their MAH or DMAH submit an application for pre-market approval to the PMDA and be approved by the PMDA before they can be registered and placed on the market.
It is worth noting that medical devices from outside Japan can only be registered in Japan by a Japanese Authorised Representative (MAH or DMAH), and that medical device manufacturers from outside Japan can only obtain the ‘Certificate of Registration as a Foreign Manufacturer of Medical Devices’ (i.e., the Certificate of Compliance with the Systematic Review).
