UKCA Registered Agent Service
The MHRA (Medical & Healthcare products Regulatory Agency) is under the jurisdiction of the Department of Health and Social Care (DHSC) in the UK.
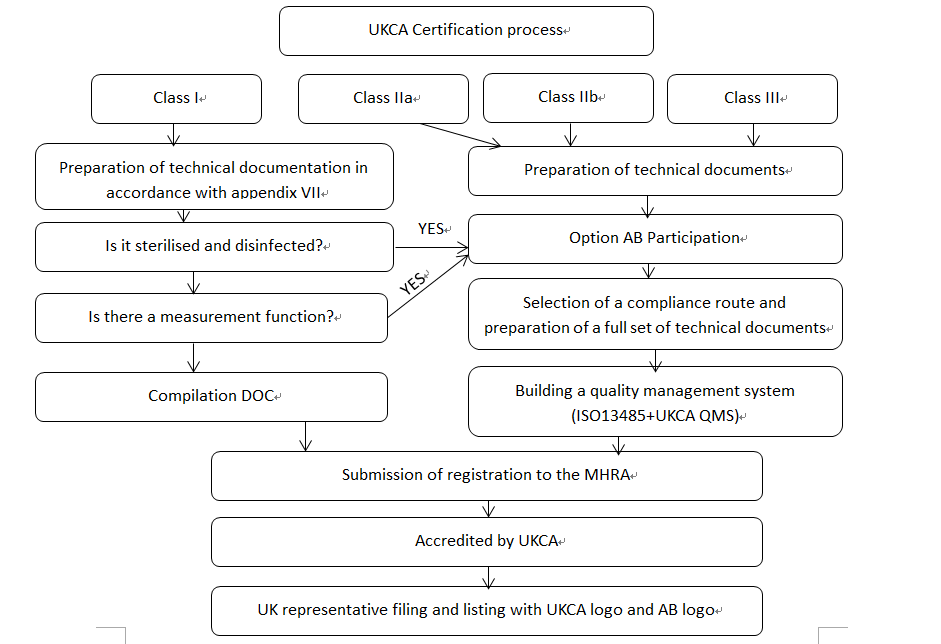
The quickest way for offshore companies to list medical devices in the UK is to convert using an existing CE MDR certificate, or to convert a certificate or part of a technical file of a medical device certification obtained in Australia, Canada, the European Union, or the United States of America, in accordance with the draft of the proposed recognition of Australian, Canadian, European Union, and U.S. medical device certifications published by the MHRA on 21 May 2024, which is expected to be implemented in 2025, and which is expected to be implemented in 2025. is expected to be implemented in 2025.
It is worth noting that: a. To apply for registration in the UK, overseas enterprises will need a UK Authorised Representative (UKRP), who will carry out legal duties for the overseas enterprises in the UK. b. If the medical device registration certification of other proposed recognised countries is used for the conversion, the UK MHRA will not issue the UKCA certification mark. c. If the overseas enterprises do not use the certification of the proposed recognised countries for the conversion in the UK, they will need to approach the UK MHRA for the conversion of the certificates or part of the technical documents of those proposed recognised countries. to the UK, they will need to approach AB ‘Approved Body’, a third party independent certification body appointed by the UK MHRA, to apply for UKCA accreditation. d. Northern Ireland is not included in the UK medical device regulatory legislation. e. The UKCA accreditation mark will not be issued to companies that do not use the accreditation of other proposed countries for conversion to the UK.
The quickest way for offshore companies to list medical devices in the UK is to convert using an existing CE MDR certificate, or to convert a certificate or part of a technical file of a medical device certification obtained in Australia, Canada, the European Union, or the United States of America, in accordance with the draft of the proposed recognition of Australian, Canadian, European Union, and U.S. medical device certifications published by the MHRA on 21 May 2024, which is expected to be implemented in 2025, and which is expected to be implemented in 2025. is expected to be implemented in 2025.
It is worth noting that: a. To apply for registration in the UK, overseas enterprises will need a UK Authorised Representative (UKRP), who will carry out legal duties for the overseas enterprises in the UK. b. If the medical device registration certification of other proposed recognised countries is used for the conversion, the UK MHRA will not issue the UKCA certification mark. c. If the overseas enterprises do not use the certification of the proposed recognised countries for the conversion in the UK, they will need to approach the UK MHRA for the conversion of the certificates or part of the technical documents of those proposed recognised countries. to the UK, they will need to approach AB ‘Approved Body’, a third party independent certification body appointed by the UK MHRA, to apply for UKCA accreditation. d. Northern Ireland is not included in the UK medical device regulatory legislation. e. The UKCA accreditation mark will not be issued to companies that do not use the accreditation of other proposed countries for conversion to the UK.
- Consult
- National Service Hotline :
0755-27391220