-
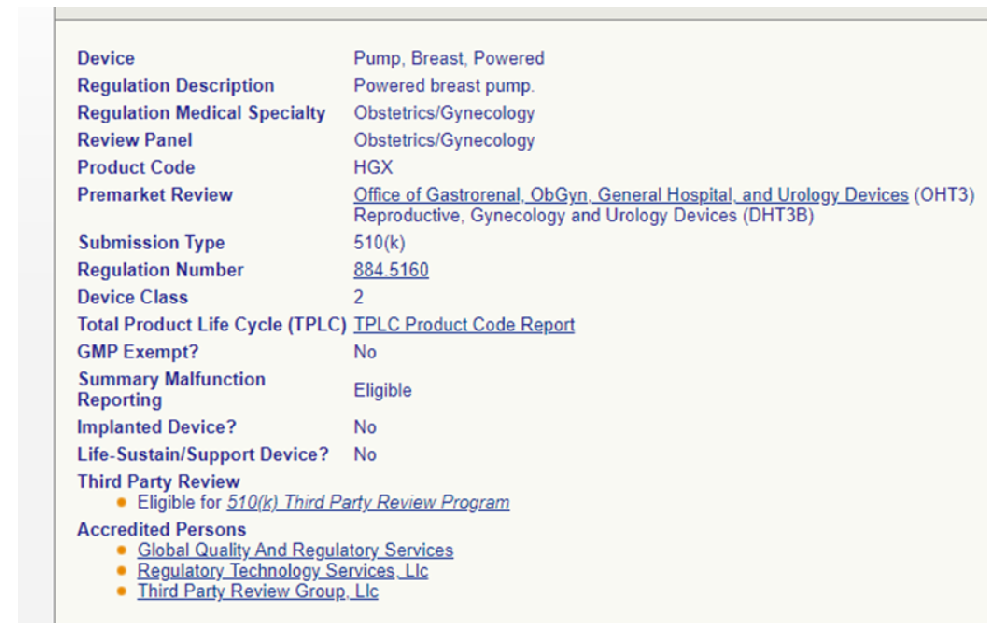
Electric Breast Pump FDA 510k Registration Zero Hair Replacement
Breast pump FDA 510K registration with zero issuance of patches, certified in 2.5 months!
388

-
Urion FDA Factory Inspection-2020
Shenzhen Urion Technology mainly produces arm and wrist electronic sphygmomanometers, infrared thermometers, the company's two sphygmomanometer products obtained 510(K) and exported to the U.S. for about a year.After receiving the FDA factory inspection notice, the client chose a well-known consulting firm to work with. The client rec···
351

-
Simzo ISO 13485:2016 Quality System Certification
Dongguan Simzo Electronics is a professional R & D, production and sales of home medical equipment company, products include electronic thermometer, infrared thermometer, ultrasonic nebuliser, compression nebuliser and so on.Based on the previous cooperation, the customer signed a long-term strategic consulting contract with our compa···
352

-
Jinghao Hearing Aid CE marking passed
Huizhou Jinghao Electronics is a company specialising in the development and production of nebulisers, hearing aids, alternating pneumatic mattresses and other medical devices.The company originally cooperated with a domestic consulting company, but the CE connection of hearing aids failed to pass the audit for three times.After the recom···
453

-
Rongfeng Class II Medical Device System Accreditation Passed
Shenzhen Rongfeng Technology Co., Ltd. was originally a trading company, and started to set up a factory due to the company's business development needs.The company mainly produces Class II active medical devices, such as electronic thermometers, infrared thermometers, blood pressure monitors and other products.After the efforts of th···
337



