
-
MDR certification is a type of EU CE certification
Under the MDR certification for medical devices, it belongs to a type of EU CE certification, which was formerly known as the EU's medical device CE certification standard. The MDR certification for medical devices was released in May 2020, which is a new version and is intended to replace the mandatory certification for EU medical device CE re···
115
-
FDA510K certification application process cycle
Medical device FDA 510 (k) registration is commonly referred to as 510 (K) registration due to its corresponding FD&C Act Chapter 510. 510 (k) is one of the main channels for medical devices to be launched in the United States, and the vast majority of Class II medical devices and local Class I and III medical devices go through this processCle···
110
-
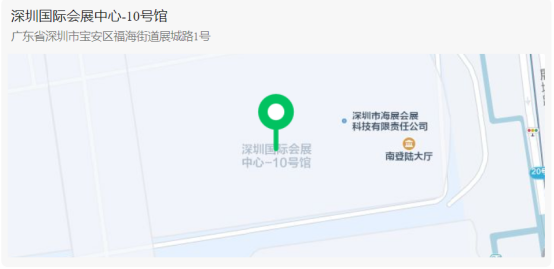
Invitation Letter for 88th CMEF Exhibition and Forum
To all dear customersThe 88th China International Medical Equipment Expo (CMEF) was grandly held from October 28th to 31st, 2023 at the Shenzhen International Convention and Exhibition Center (Bao'an Hall).The Renni booth is located at booth 10C52 (next to the elevator on the right side of the entrance hall) in Hall 10 of the Convention and Exh···
108

-
Renni's participation in the 88th China International Medical Device (Autumn) Expo (CMEF) has been successfully concluded
The 88th China International Medical Equipment Expo (CMEF) was successfully concluded on October 31, 2023 at the Shenzhen International Convention and Exhibition Center (Bao'an Hall).Renni has been invited to participate in the exhibition, and I am very grateful to the customers/colleagues/friends who were present for their strong support. I al···
106


